__________
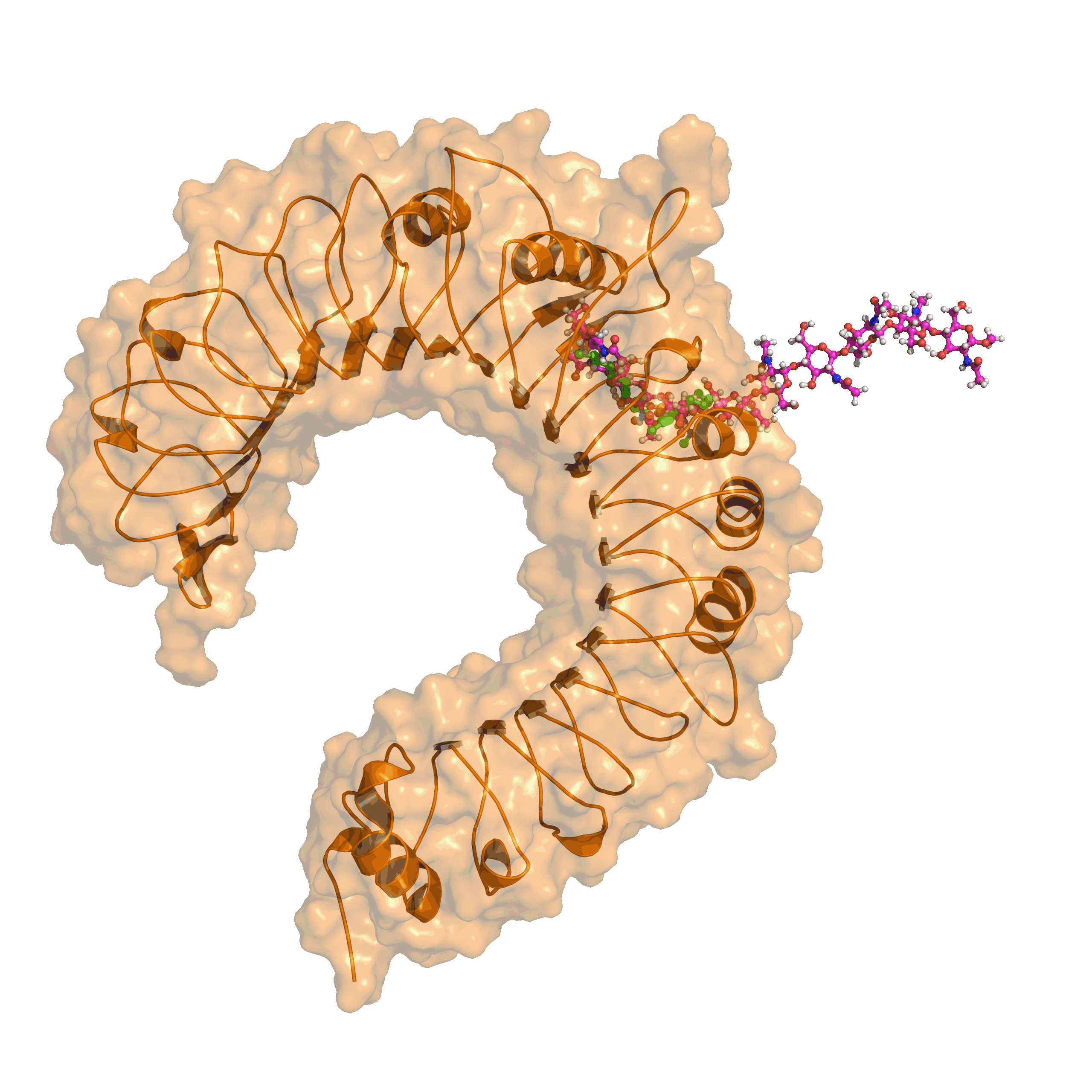
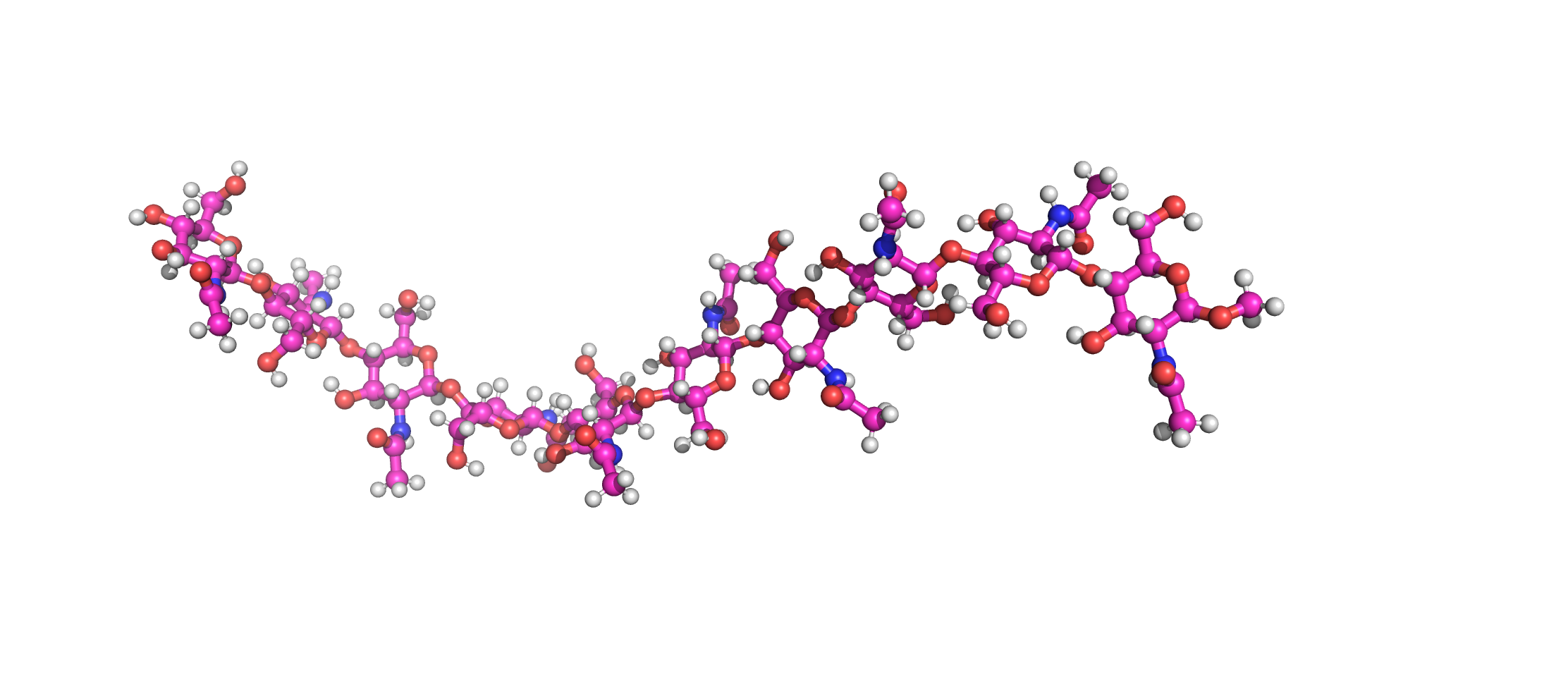
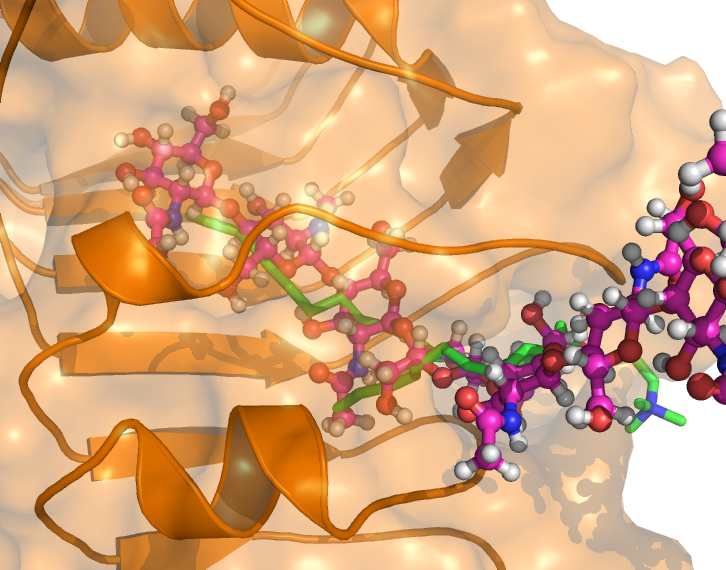
End of March 2022, the Senate of the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) established the Priority Programme “Code𝛘 – Chitin, chitosan and chito-oligosaccharides and their interaction with proteins of the extracellular matrix and cellular signaling” (SPP 2416)”. This programme is designed to run for six years, and the call invited proposals from researchers working in this field to join for the first three-years funding period, which has started in October 2023.
Coordinator: Prof. Dr. Hans Merzendorfer, Dept. of Chemistry-Biology, University of Siegen
Extended Board
Advisory Board (Mercator Fellows)
_______
Hosted by Prof. Dr. Holger Gohlke, the 2nd Code𝛘 Summer School took place from 14th to 16th July 2025 at…
[16th ICCC/9th SIAQ] – Early bird registration 30th July, 2025
The Iberoamerican Chitin Society and the International Federation of Chitin & Chitosan Societies are pleased to invite you to the…
[APCCS 2025] – Abstract submission deadline has been extended to May 15th, 2025
The 14th Asia-Pacific Chitin and Chitosan Symposium (14th APCCS 2025) will be held from August 26th to 29th, 2025 at…
Code𝛘 Annual Retreat brought together Chitin/Chitosan researchers in Münster
The Code𝛘 Annual Retreat 2025 took place at the University of Münster on February 24th and 25th, 2025, hosted by…
Code𝛘 Annual Retreat 2025 in Münster – Registration Now Open
Our Code𝛘 Annual Retreat 2025 will take place on February 24th and 25th, hosted by Prof. Bruno Moerschbacher and his…
_____________
In this area, we will provide information on the participating projects funded in the scope of this priority programme.