______________
Analysis of chito-protein interactions
About the Project
This service project provides equipment and expertise for the determination of binding properties of chito-protein and protein-protein interactions by surface-coated quart crystal microbalance-dissipation (QCM-D) chips and grating-coupled interferometry (GCI).
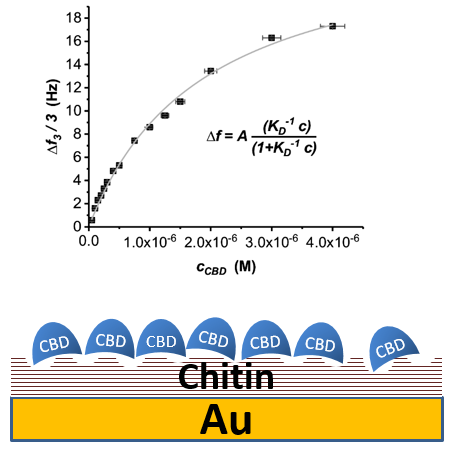
As chitin and chitosan (C/CS) polymers are insoluble fibrillar materials, accurate measurement of kinetic parameters and determination of equilibrium constants for C/CS interacting proteins is challenging. We have developed a rapid QCM-D based method to determine binding constants of chitin-binding proteins on chitin-coated gold surfaces (Vogt et al, 2018, Analyst 143, 5255-5263). For this purpose, alpha-chitin was first trimethylsilylated and coated onto the sensor chips. After desilylation, regular fibril‑like structures with a typical center-to-center spacing of 85 nm were observed by atomic force microscopy. Using different setups and data evaluation methods for QCM-D measurements, we determined kon and koff, and calculated the KD values for binding of a recombinant CBD from Bacillus circulans chitinase A1. This bacterial CBD has been analyzed in detail before and thus served as a well-characterized reference for determining chitin-binding properties by QCM-D measurements. The obtained KD values were in good agreement with those measured for other bacterial CBDs usually ranging between 1 to 10 µM. We will provide this experimental approach in Service Project 3 to determine unknown binding affinities of various chitin-binding domains/proteins to crystalline alpha-chitin fibers. We further will extend this method to allow measurements for the binding to beta-chitin fibers, and to chitins and chitosans with various degrees of acetylation and polymerization.
In this service project, we will also offer measurements of protein interactions using grating coupled interferometry (GCI), which is based on the highly sensitive waveguide interferometry principle. For this purpose, the GCI device will be requested as a central device of the consortium. The system uses interchangeable, reusable chips based on a robust microfluidic system that prevents clotting and allows measurements in complex solutions, as it provides excellent signal-to-noise ratios due to an extended light-to-sample interaction length. Functionalized chips are available with different coatings to bind one of the interaction partners. Therefore, interaction measurement are possible in undiluted plasma and serum, crude membrane extracts and complex matrices. This allows high-resolution determination of binding parameters with reliable kinetics below 1pg/mm2 without the need of data averaging. In addition, the measurements of the interaction of very small ligands (>1000 Da, including COS) with large proteins or particles (e.g. liposomes) are possible. The kinetic range to be determined is very broad and ultra-fast transition times (reliable off-rates of up to 10 sec-1). The system will be extended for high throughput measurements at single ligand concentrations using the waveRAPID Kinetics™ in the second funding period. In addition, reusable WAVEchips will be developed that are equipped with layers of different chemically defined chito-oligomers (coupled via polyethylene glycol linkers using thiol chemistry, in collaboration with SP).
In addition to determination of biding parameters , we provide the following support:
- Non-radioactive assays for measuring chitin synthesis
- Fluorescent recombinant chitin-binding proteins
- Production and pruficiation of recombinant proteins in bacterial, fungal or insect cells
References
S. Vogt, M. Kelkenberg, T Nöll, B. Steinhoff, H. Schönherr, H. Merzendorfer, G. Nöll. “Rapid determination of binding parameters of chitin binding domains using chitin-coated quartz crystal microbalance sensor chips.” Analyst 143, 5255-63 (2018)
C. Hong, N.J. Byrne, B. Zamlynny, S. Tummala, L. Xiao, J.M. Shipman, A.T. Partridge, C. Minnick, M.J. Breslin, M.T. Rudd, et al. “Structures of active-state orexin receptor 2 rationalize peptide and small-molecule agonist recognition and receptor activation.” Nat Commun 12, 815. DOI: https://doi.org/10.1038/s41467-021-21087-6 (2021)
H. Jankovics, B. Kovacs, A. Saftics, T. Gerecsei, E. Toth, I. Szekacs, F. Vonderviszt, R. Horvath. “Grating-coupled interferometry reveals binding kinetics and affinities of Ni ions to genetically engineered protein layers”. Sci Rep 10, 22253. DOI: https://doi.org/10.1038/s41598-020-79226-w (2020)
Ö. Kartal, F. Andres, M.P. Lai, R. Nehme, R & K. Cottier. ” waveRAPID- A Robust Assay for High-Throughput Kinetic Screens with the Creoptix WAVE system.” SLAS DISCOVERY: Advancing the Science of Drug Discovery, 24725552211013827. DOI: https://doi.org/10.1101/2021.02.05.42987 (2021)
P. Jimenez Sandoval, J. Santiago. “In vitro analytical approaches to study plant ligand-receptor interactions”, Plant Physiology, DOI: https://doi.org/10.1104/pp.19.01396 (2020)

Principal Investigators



